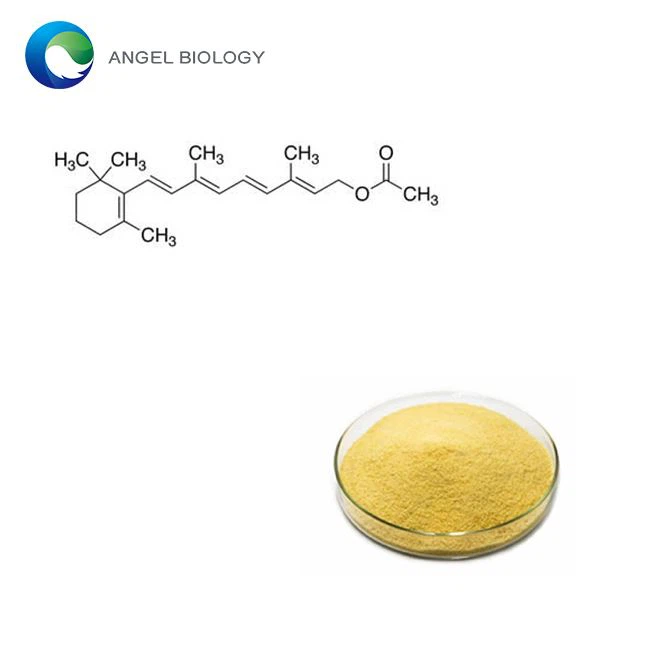
Why Pharmaceutical Lycopene Requires Nano-Encapsulation
The journey from raw tomatoes to pharmaceutical-grade lycopene powder is not as straightforward as one might think. Lycopene, in its natural state, poses significant challenges for pharmaceutical applications. Its poor water solubility and low bioavailability make it difficult for the body to absorb and utilize effectively. This is where the innovative process of nano-encapsulation comes into play.
Nano-encapsulation is a cutting-edge technique that involves encasing lycopene molecules within microscopic particles. This process serves several crucial purposes:
- Enhanced Bioavailability: By reducing the particle size to nanoscale, the surface area of lycopene increases dramatically. This allows for better absorption in the gastrointestinal tract, significantly boosting its bioavailability.
- Improved Stability: Lycopene is notoriously sensitive to light, heat, and oxygen. Nano-encapsulation provides a protective shell that shields the compound from these degradative factors, extending its shelf life and preserving its potency.
- Controlled Release: The nano-encapsulation process can be tailored to achieve controlled release of lycopene in the body. This means the compound can be delivered to specific target sites more effectively, enhancing its therapeutic potential.
- Increased Solubility: By encapsulating lycopene within water-soluble materials, its solubility can be greatly improved. This is particularly important for pharmaceutical applications where aqueous solutions are often preferred.
The nano-encapsulation process typically involves sophisticated techniques such as emulsification, spray-drying, or supercritical fluid technology. These methods require precise control over various parameters like temperature, pressure, and particle size distribution to ensure consistent quality of the final product.


Strict Quality Control in Medical-Grade Lycopene Production
The production of pharmaceutical-grade lycopene powder is subject to stringent quality control measures. These rigorous standards are essential to ensure the safety, efficacy, and consistency of the final product. Let's examine some of the key aspects of quality control in medical-grade lycopene production:
- Raw Material Sourcing: The process begins with carefully selecting high-quality tomatoes or other lycopene-rich sources. These raw materials undergo thorough testing for pesticide residues, heavy metals, and microbial contamination.
- Extraction Purity: The extraction process is optimized to isolate lycopene with minimal impurities. Advanced chromatography techniques are employed to ensure the highest level of purity.
- Particle Size Analysis: Given the importance of nano-encapsulation, precise control over particle size is crucial. Sophisticated instruments like dynamic light scattering (DLS) or laser diffraction are used to measure and verify the size distribution of lycopene nanoparticles.
- Chemical Characterization: High-performance liquid chromatography (HPLC) and mass spectrometry are utilized to confirm the chemical identity and purity of the lycopene powder. These methods can detect even trace amounts of impurities or degradation products.
- Stability Testing: The lycopene powder undergoes extensive stability testing under various environmental conditions. This ensures that the product maintains its potency and quality throughout its shelf life.
- Microbial Testing: Rigorous microbiological testing is conducted to ensure the powder is free from harmful bacteria, yeasts, and molds.
- Bioavailability Studies: In vitro and in vivo studies are often performed to verify the enhanced bioavailability of the nano-encapsulated lycopene compared to its non-encapsulated form.
These quality control measures are not just one-time checks but are integrated throughout the production process. Continuous monitoring and documentation ensure that every batch of pharmaceutical-grade lycopene powder meets the highest standards of quality and consistency.
From Raw Tomatoes to Lab-Tested Lycopene Powder
The transformation of raw tomatoes into pharmaceutical-grade lycopene powder is a multi-step process that combines traditional extraction methods with cutting-edge technology. Let's walk through the journey:
- Harvesting and Selection: The process begins with carefully selecting ripe tomatoes at their peak lycopene content. These tomatoes are harvested and quickly processed to prevent degradation.
- Washing and Preparation: The tomatoes are thoroughly washed to remove any surface contaminants. They are then crushed or pureed to break down the cell walls and release the lycopene.
- Extraction: The lycopene is extracted from the tomato puree using food-grade solvents. This step is carefully controlled to maximize lycopene yield while minimizing the extraction of unwanted compounds.
- Solvent Removal: The solvent is then removed through evaporation under controlled temperature and pressure conditions. This step is crucial to ensure no residual solvent remains in the final product.
- Purification: The crude lycopene extract undergoes multiple purification steps, including crystallization and recrystallization. These processes help remove impurities and increase the concentration of lycopene.
- Nano-encapsulation: The purified lycopene is then subjected to nano-encapsulation. This may involve techniques like high-pressure homogenization or emulsification to create nano-sized particles.
- Drying: The nano-encapsulated lycopene solution is carefully dried, often using spray-drying or freeze-drying techniques. These methods preserve the integrity of the nanoparticles while converting the solution into a fine powder.
- Milling and Sieving: The dried powder may undergo further milling and sieving to ensure a uniform particle size distribution.
- Lab Testing: The final lycopene powder undergoes extensive laboratory testing to verify its purity, potency, and compliance with pharmaceutical standards.
- Packaging: Once approved, the pharmaceutical-grade lycopene powder is packaged under controlled conditions to protect it
 from light, moisture, and oxygen.
from light, moisture, and oxygen.
Throughout this process, strict adherence to Good Manufacturing Practices (GMP) is maintained. Every step is meticulously documented, and quality checks are performed at multiple stages to ensure the highest standards of pharmaceutical production are met.
The result of this complex process is a high-quality, pharmaceutical-grade lycopene powder that offers enhanced bioavailability and stability compared to its natural form. This lycopene powder can be used in various pharmaceutical applications, from nutritional supplements to potential therapeutic formulations.
Conclusion
The production of pharmaceutical-grade lycopene powder is a testament to the intersection of nature and advanced technology. By harnessing the power of nano-encapsulation and adhering to stringent quality control measures, we can transform a simple tomato into a potent, bioavailable form of lycopene with immense potential for human health.
As research continues to unveil the benefits of lycopene, the demand for high-quality, pharmaceutical-grade lycopene powder is likely to grow. The complex process of its production ensures that we can harness the full potential of this remarkable compound, opening new avenues in nutrition and medicine.
If you're interested in learning more about our pharmaceutical-grade lycopene powder or other natural ingredients for health and wellness applications, we invite you to reach out to us. At Angelbio, we're committed to providing high-quality, innovative solutions for the nutritional supplement, cosmetic, and pharmaceutical industries. Our team of experts is ready to assist you in finding the perfect ingredients for your products. Contact us today at angel@angelbiology.com to discuss how we can support your formulation needs with our premium, scientifically-backed ingredients.
References
1. Johnson, E. J., & Krinsky, N. I. (2009). Carotenoids and coronary heart disease. In Carotenoids (pp. 287-300). Birkhäuser Basel.
2. Rao, A. V., & Rao, L. G. (2007). Carotenoids and human health. Pharmacological research, 55(3), 207-216.
3. Gartner, C., Stahl, W., & Sies, H. (1997). Lycopene is more bioavailable from tomato paste than from fresh tomatoes. The American journal of clinical nutrition, 66(1), 116-122.
4. Zu, K., Mucci, L., Rosner, B. A., Clinton, S. K., Loda, M., Stampfer, M. J., & Giovannucci, E. (2014). Dietary lycopene, angiogenesis, and prostate cancer: a prospective study in the prostate-specific antigen era. Journal of the National Cancer Institute, 106(2), djt430.